Anaveon doses first patient in a Phase I/II study to evaluate the safety, dosing and clinical activity of ANV419 in patients with solid tumors
— ANV419 is a powerful and selective interleukin-2 (IL-2) agonist targeting cancer —
Basel, June 24, 2021 – Anaveon, a clinical stage, immuno-oncology company, today announced that it has successfully dosed the first patient in a Phase I/II open label study of ANV419, a powerful and selective interleukin-2 (IL-2) agonist as a monotherapy in patients with advanced solid tumors. ANV419 has been designed to overcome known challenges with tolerability and selectivity of recombinant human IL-2.
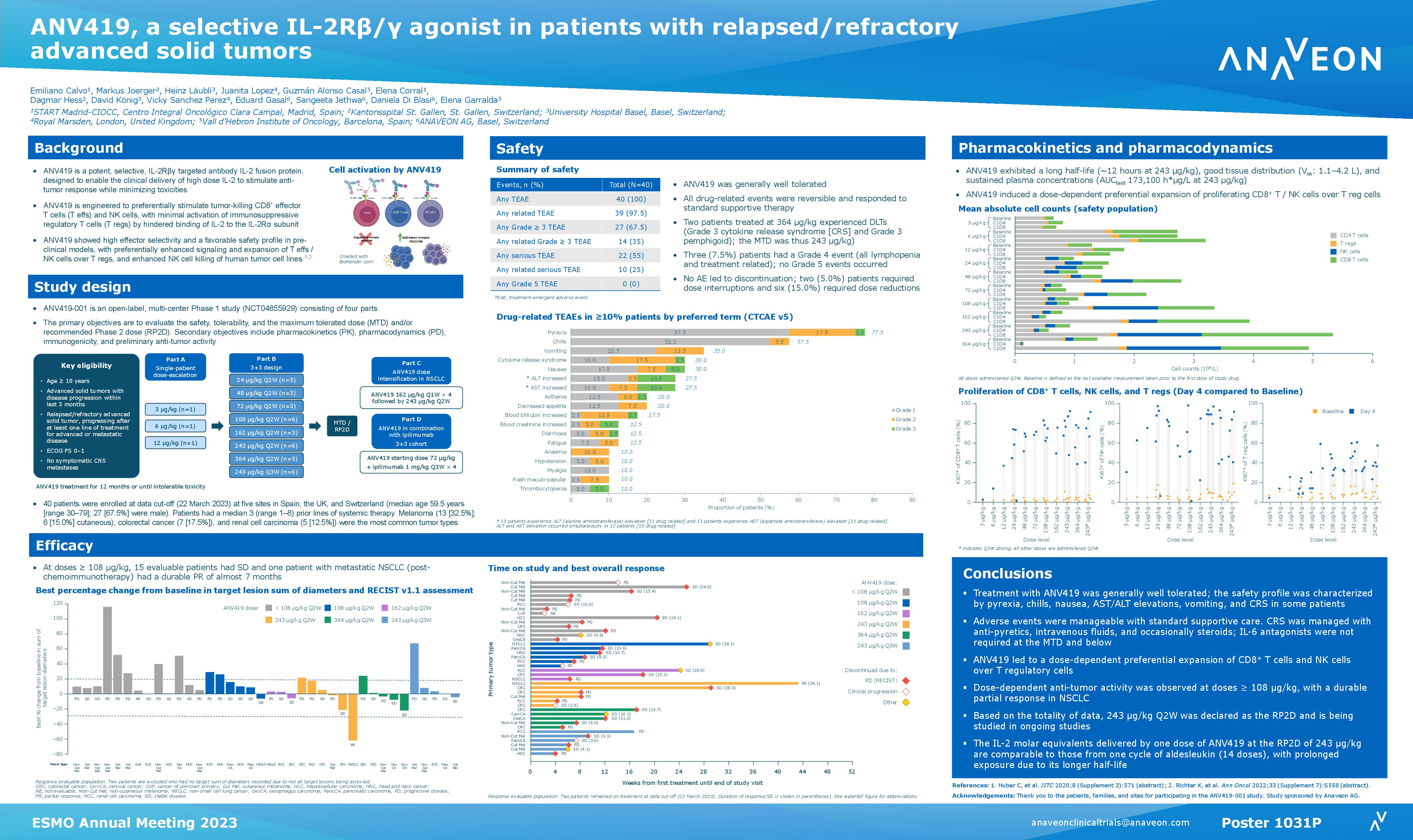
The study is an open-label, multi-center Phase I/II trial evaluating the safety, tolerability, dose finding and clinical efficacy of ANV419 in patients with advanced solid tumors. Recruitment is ongoing in Spain. Link to the study.
“Dosing our first patient represents an important validation of our approach and is a significant milestone for Anaveon,”said Dr Christoph Bucher, Chief Medical Officer of Anaveon. “We have initiated a multi part, first-in-human dose finding study of ANV419 as a monotherapy in patients with solid tumors. Our compound has excellent tolerability in non-human primates and marked efficacy in a variety of tumor models and elicits strong and selective proliferation of effector cells in multiple settings.”
Martin Murphy, Chief Executive of Syncona Investment Management Limited, added:“We are encouraged by Anaveon’s strong progress since our first investment in the company in 2019. The first patient dosing comes off the back of pre-clinical data which has shown that ANV419 has the potential to be a best-in-class asset. Whilst not without risk, the clinical data generated by the company will be a critical driver of value and there is a fast timeline for the company to show differentiation against competing products. We are excited about its potential.”
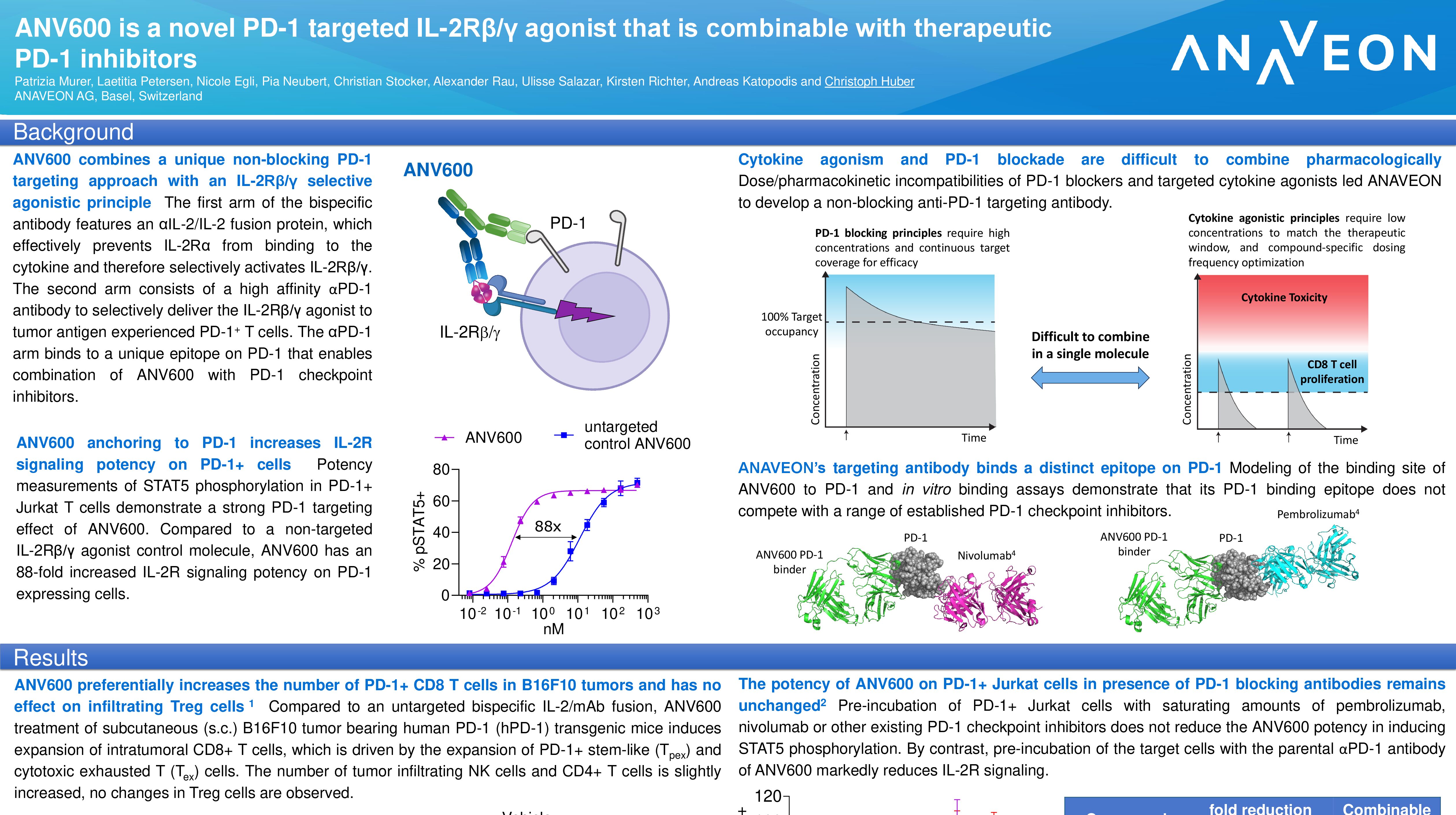
ANV419 is a novel IL-2/anti-IL-2 fusion protein with preferential signaling through the IL- 2 beta/gamma receptor. It has antibody-like pharmacology, behavior and stability and selectively stimulates the proliferation of CD8 T cells and NK cells without promoting the expansion of Regulatory T cells (Tregs). In non-human primate studies, ANV419 demonstrated excellent tolerability, a highly favorable safety profile and pharmacokinetics with strong in-vivo expansion of NK and CD8 T cells but not Tregs. No significant changes in body weight or blood pressure were seen at any dose during the entire study period and no signs of capillary leak syndrome were observed. Anaveon expects to report initial clinical safety and pharmacokinetic (PK)/ pharmacodynamic (PD) data by the end of 2021.
Anaveon was founded in December 2017 by Andreas Katopodis, previously Director at the Autoimmunity, Transplantation & Inflammation Group at the Novartis Institutes for BioMedical Research and Onur Boyman, Professor and Chair in the Department of Immunology at the University of Zurich. The Company is developing selective IL-2 Receptor Agonists, which have the potential to therapeutically enhance a patient’s immune system to respond to tumors. In the body, human IL-2 stimulates a type of immune cell, called a T-cell, to multiply and become activated. Activated T-cells are able to attack tumors and, consistent with this approach, human IL-2 is already approved as a therapeutic for the treatment of metastatic melanoma and renal cancer; however, due to lack of specificity, human IL-2 has severe, dose-limiting side effects and a short half-life that requires frequent infusions. The lead compound, ANV419, is designed to preferentially signal through the IL-2 beta/gamma receptor and therefore overcome known challenges of human IL-2. This novel type of therapeutic, if approved, could potentially have a wide utility in oncology, including in combination with cell therapies, vaccines, checkpoint inhibitors and radiotherapy.
ENDS
Enquiries
JW Communications
Julia Wilson
Tel: +44 (0)7818 430877
Email: julia.wilson@anaveon.com
About Anaveon:
Anaveon is a clinical stage, biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.