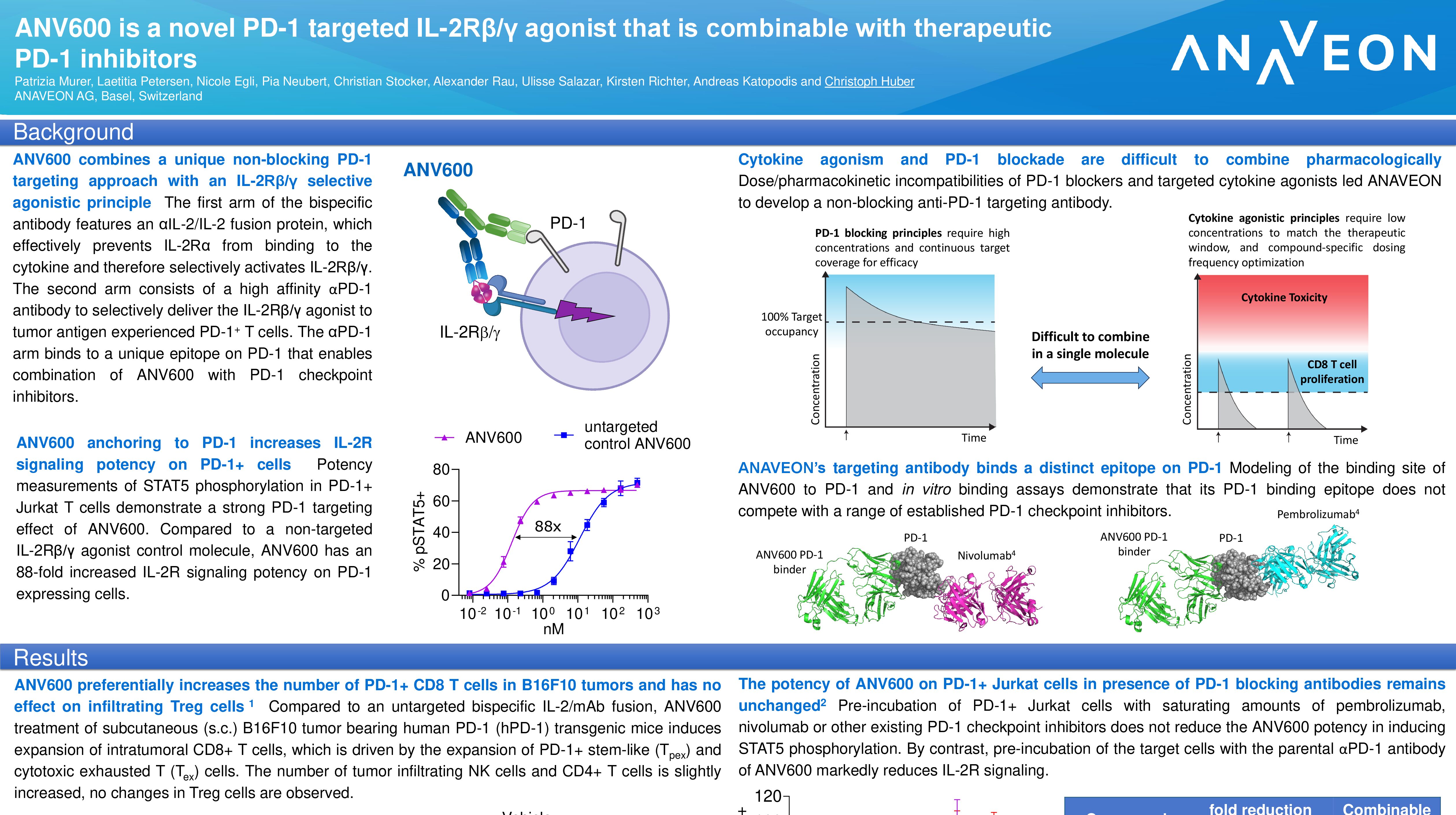
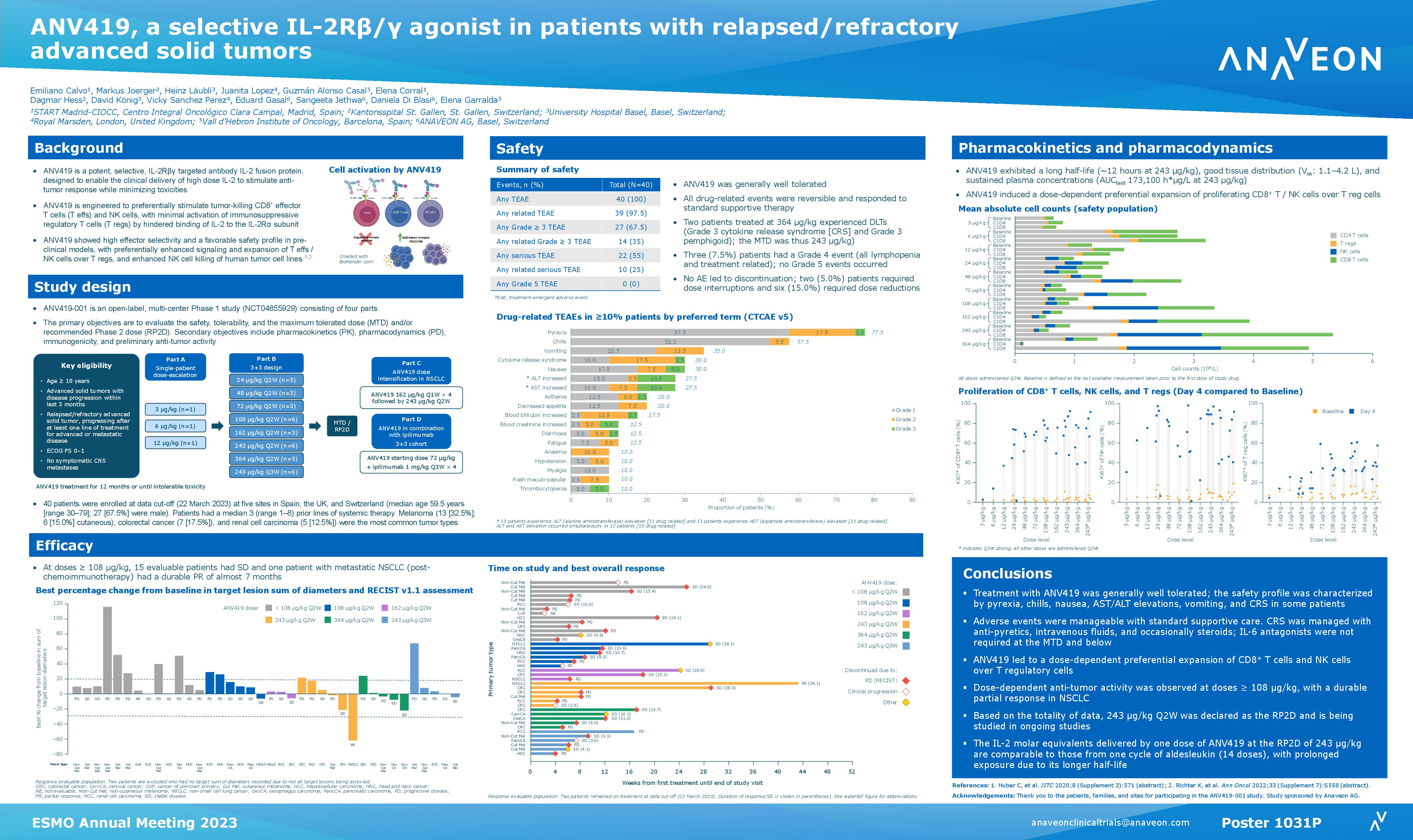
Anaveon Announces FDA Safe to proceed letter for Investigational New Drug (IND) Application for its no-alpha IL-2 agonist, ANV419

Basel, June 9, 2022 – Anaveon, a clinical-stage immuno-oncology company, today announced that the U.S. Food and Drug Administration (FDA) has cleared the IND application for ANV419-101, a Phase I/II mono treatment dose confirmation and combination dose-finding, global study, in patients with advanced cutaneous melanoma.
ANV419, a potent IL-2Rbg agonist, preferentially proliferates CD8 and NK cells over regulatory T cells. The safety profile seen to date will allow for the administration of doses inducing maximal pharmacodynamic effects. The most frequent drug related AEs seen in an ongoing trial include mild Grade 1 chills and low-grade fever a few hours after dosing, which resolved with antipyretic treatment.
“We are excited to bring ANV419 to patients who are most likely to benefit from a truly IL-2Rbg-selective high-dose IL-2 approach,” said Christoph Bucher, Chief Medical Officer at Anaveon. “We look forward to expanding our clinical program to the US and maximizing the therapeutic potential of ANV419 for patients suffering from cancer.”
About the Phase I/II Clinical Trial
The clinical trial is a Phase I/II multiple arm, open-label study in patients with unresectable or metastatic cutaneous melanoma. The study will be a sequential, multi-part clinical trial to evaluate the safety and efficacy of different monotherapy doses of ANV419, as well as in combination with anti-PD1 or anti-CTLA4. Up to 130 patients will be enrolled in the clinical trial.
ENDS
Enquiries
JW Communications
Julia Wilson
Tel: +44 (0)7818 430877
Email: julia.wilson@anaveon.com
About Anaveon:
Anaveon is a clinical-stage biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.