Anaveon announces poster presentation at the 2023 European Society for Medical Oncology Annual Meeting
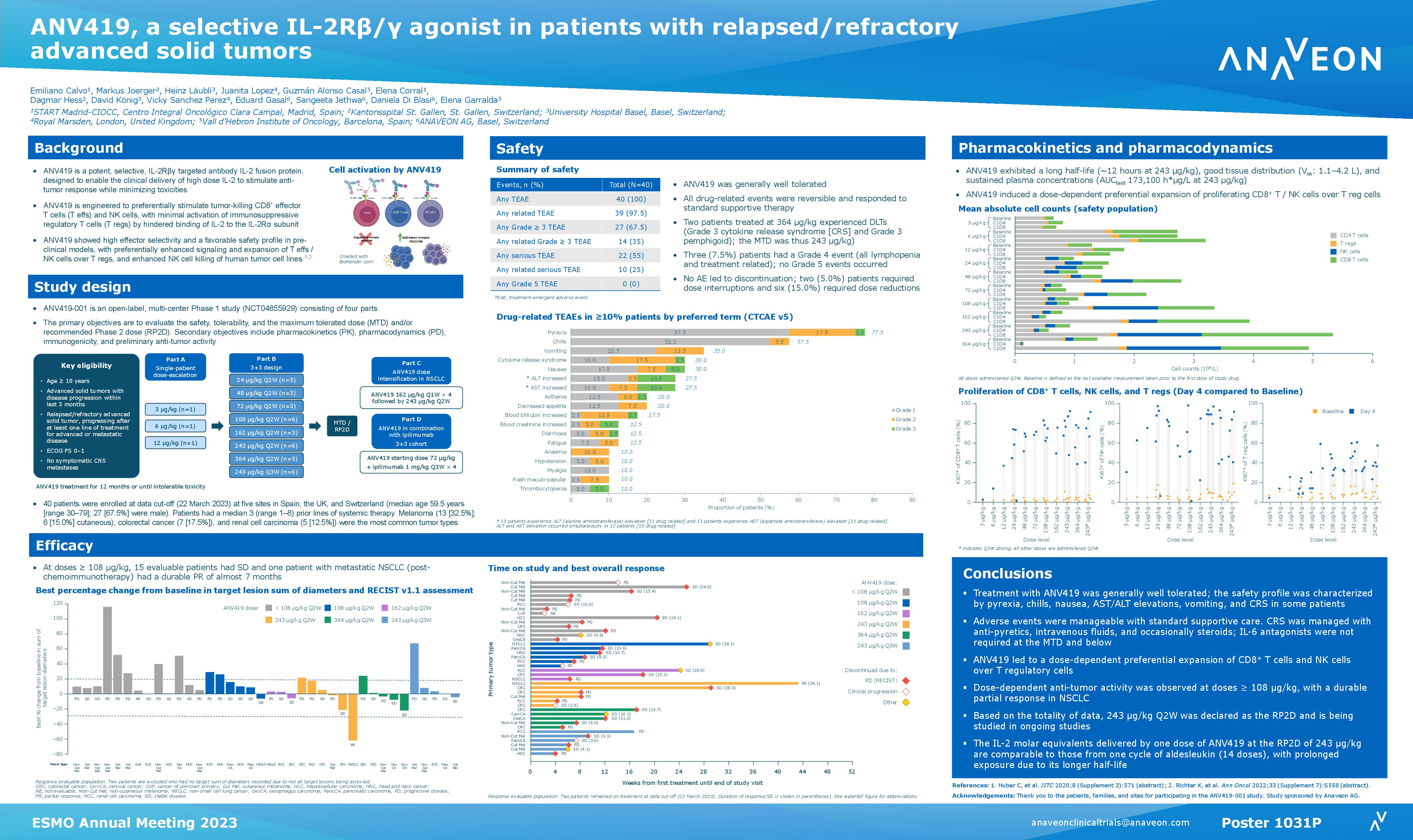
— ANV419 243 µg/kg selected as recommended Phase 2 dose for solid tumors —
Basel, October 16, 2023 – Anaveon, a clinical stage, immuno-oncology company, today announced that it will present a poster on its lead program ANV419 at the European Society of Medical Oncology (ESMO) annual meeting being held from Friday October 20, 2023, to Tuesday, October 24, 2023, in Madrid, Spain.
ANV419 is a potent and selective IL-2Rβ/γ agonist, designed to enable the delivery of high-dose IL-2 to patients in a safe way. ANV419-001 is a first-in-human, Phase 1 dose escalation study of intravenous single agent ANV419 in patients with advanced solid tumors. Overall, ANV419 was well-tolerated, with all events being self-limiting, reversible, or manageable with standard supportive care. ANV419, at a dose of 243 μg/kg every two weeks (Q2W), was determined as the maximum tolerated dose (MTD) and the recommended Phase 2 dose (RP2D). At this dose, PK modeling shows that one injection of ANV419 delivers more IL-2 exposure than a full cycle of high dose aldesleukin.
The abstract is available on the ESMO wesite, and the accompanying poster will be on display on the ESMO 2023 meeting platform as well as on Anaveon’s website from October 23, 2023 (download poster here).
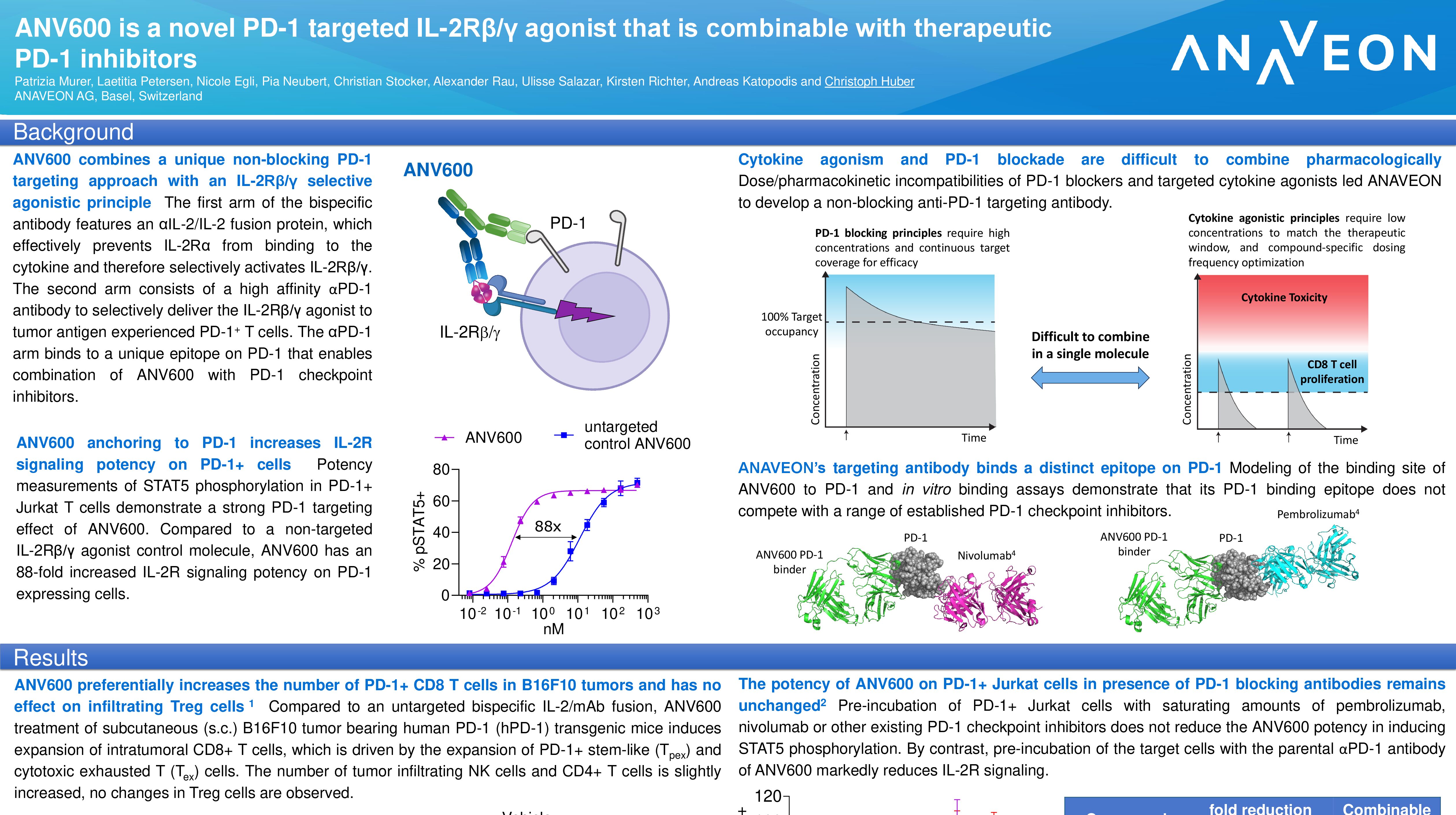
Poster # 1031P – ANV419, a selective IL-2Rβ/γ agonist in patients with relapsed/refractory advanced solid tumors
Authors: E. Calvo, M. Joerger, H. Läubli, J. Lopez, G. Alonso Casal, E. Corral, D. Hess, D. König, V. S. Perez, E. Gasal, S. Jethwa, D. Di Blasi, E. Garralda
The Poster will be presented on Monday, October 23, 2023, between 12:00 pm and 1:00 pm (CEST).
Anaveon is developing selective cytokine receptor agonists with the potential to therapeutically enhance a patient’s immune system to respond to tumors. ANV419, currently in Phase 2 studies in multiple cancer indications, is designed to preferentially signal through the IL-2 beta/gamma receptor resulting in strong proliferation of effector cells in patients. The follow-on compound, ANV600, targets the selective IL-2 receptor moiety to tumor infiltrating lymphocytes cells and may have therapeutic benefit in less immunogenic tumors. These novel types of therapeutics, if approved, could potentially have a wide utility in oncology, including in combination with cell therapies, vaccines, checkpoint inhibitors and radiotherapy.
ENDS
Enquiries
JW Communications
Julia Wilson
Email: Julia.Wilson@anaveon.com
Tel: +44 7818 430877
About Anaveon
Anaveon is a clinical stage, biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.