Anaveon announces publication of ANV419 Phase I clinical data in the Journal for ImmunoTherapy of Cancer
— ANV419 delivers high dose IL-2 to patients with a good safety and tolerability profile —
Basel, December 14, 2023 – Anaveon, a clinical stage, immuno-oncology company, today announced the publication of updated clinical data from the ongoing Phase I study of ANV419 in patients with advanced solid tumors in The Journal for ImmunoTherapy of Cancer (JITC). The paper titled “Phase 1 first-in-human dose-escalation study of ANV419 in patients with relapsed/refractory advanced solid tumors,” can be accessed here.
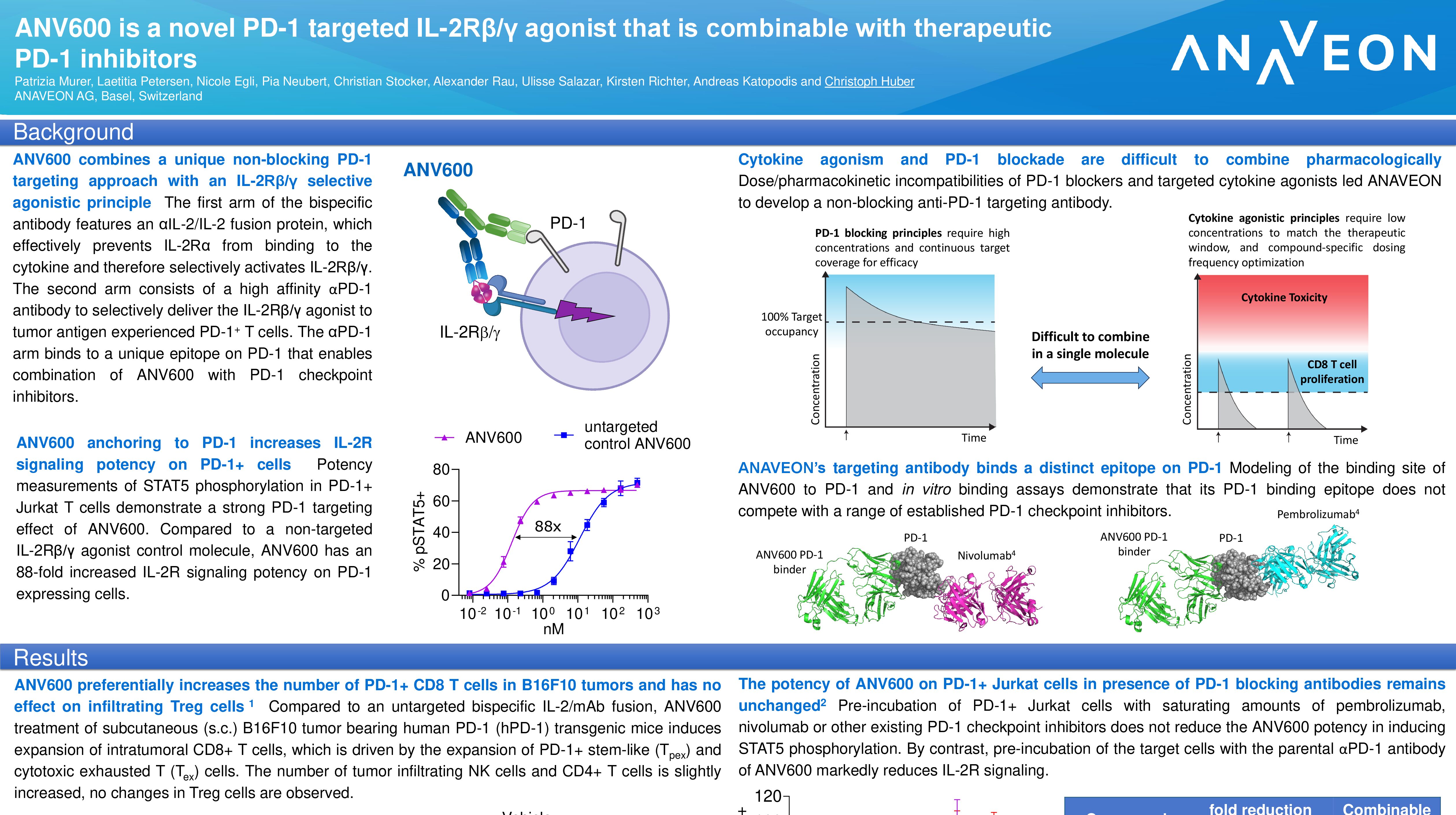
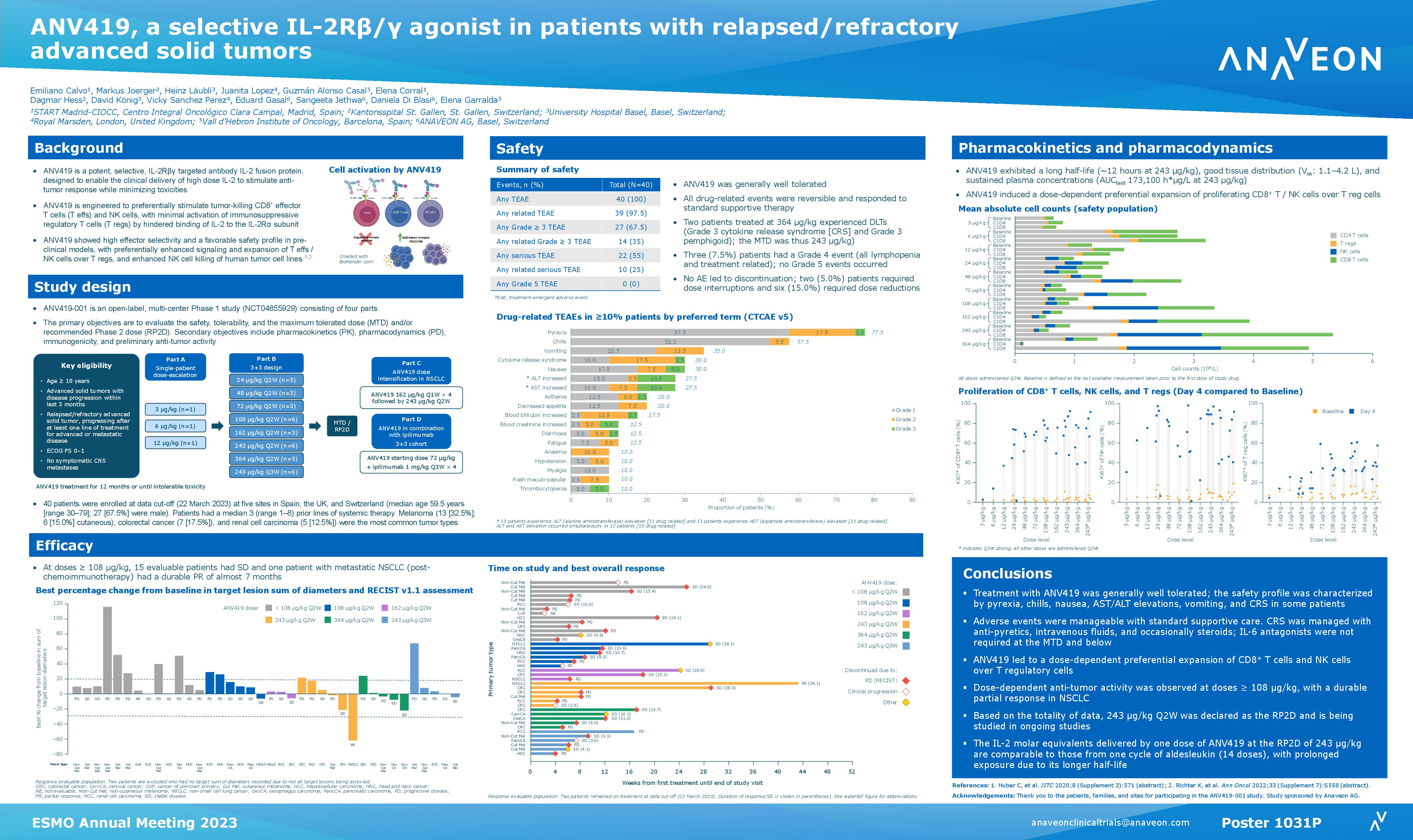
ANV419 is a powerful and selective IL-2 agonist, which has been specifically designed to enable the delivery of high dose IL-2 to patients, with a favourable safety and tolerability profile. As of the data cut-off of 31 March 2023, 40 adult patients with advanced solid tumors and progression after ≥1 previous lines of systemic therapy were enrolled and received at least one dose of ANV419. The monotherapy was delivered as an intravenous infusion once every 2 weeks at doses up to 364 µg/kg.
ANV419 was generally well tolerated, and drug-related adverse events were manageable, reversible, and responsive to supportive care therapy. The most common drug-related adverse events were low grade fever, chills, vomiting, cytokine release syndrome and nausea. Transient and self-limiting lymphopenia was observed in all patients due to lymphocyte redistribution. No patient withdrew from the study due to AEs and no dose limiting toxicities were observed up to and including 243 µg/kg.
ANV419 showed anti-tumor activity in a heavily pre-treated patient population with advanced solid tumors. At ANV419 doses ≥108 µg/kg, 64% of patients achieved at least disease stabilization and one durable response in a patient with NSCLC were observed.
Based on the totality of data, 243 µg/kg every two weeks was established as the recommended Phase 2 (RP2D) dose and is being evaluated in ongoing studies. The IL-2 molar equivalents delivered by one dose of ANV419 at the RP2D of 243 µg/kg are comparable to those from one cycle of aldesleukin (14 doses), with prolonged exposure due to its longer half-life.
In summary, in this heavily pre-treated population, at doses of up to 243 µg/kg, the maximum tolerated dose and RP2D, ANV419 was well tolerated and showed signs of anti-tumor activity in a heavily pre-treated patient population with advanced solid tumors.
Markus Joerger, MD, PhD at the Department of Medical Oncology & Hematology, Cantonal Hospital, St. Gallen, Switzerland and first author of the manuscript said, “These clinical data continue to be encouraging with ANV419 having the potential to become a component of therapy for patients with cancer.”
Anaveon is developing selective cytokine receptor agonists with the potential to therapeutically enhance a patient’s immune system to respond to tumors. ANV419, currently in Phase II studies in multiple cancer indications, is designed to preferentially signal through the IL-2 beta/gamma receptor resulting in strong proliferation of effector cells in patients. The follow-on compound, ANV600, targets the selective IL-2 receptor moiety to intratumoral effector cells and may have therapeutic benefit in less immunogenic tumors. These novel types of therapeutics, if approved, could potentially have a wide utility in oncology, including in combination with checkpoint inhibitors, cell therapies, vaccines, and radiotherapy.
ENDS
Enquiries:
JW Communications
Julia Wilson
Email: Julia.Wilson@anaveon.com
Tel: +44 7818 430877
About Anaveon:
Anaveon is a clinical stage, biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.