Anaveon announces poster presentation at the 2021 Society for Immunotherapy of Cancer Annual Meeting
— ANV419 demonstrates highly promising safety profile —
Basel, November 12, 2021 – Anaveon, a clinical stage, immuno-oncology company, today announced that it will present a poster on its lead program ANV419 at the Society for Immunotherapy of Cancer’s (SITC) 36th Annual Meeting being held from Wednesday, November 10, 2021 to Sunday, November 14, 2021.
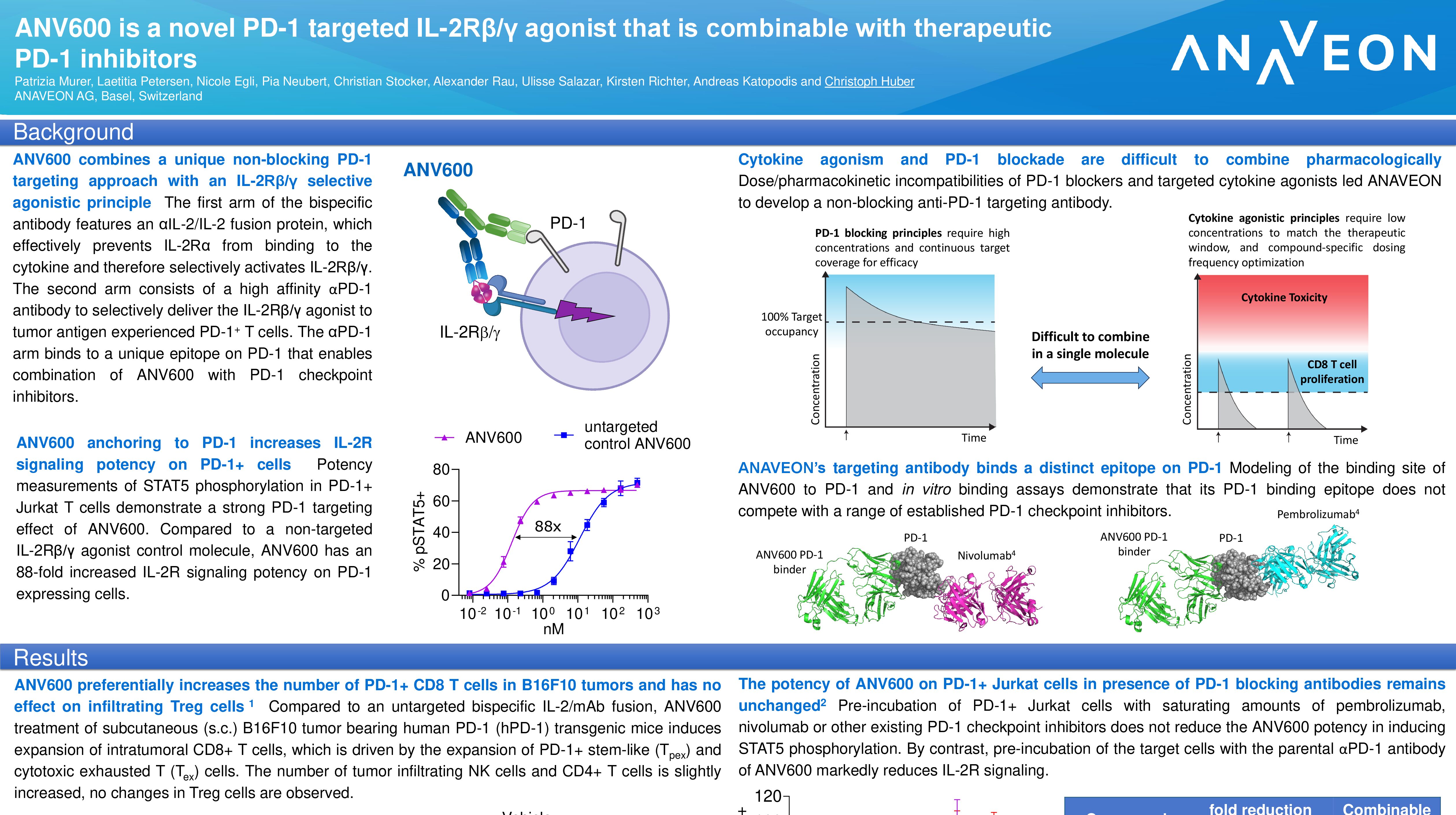
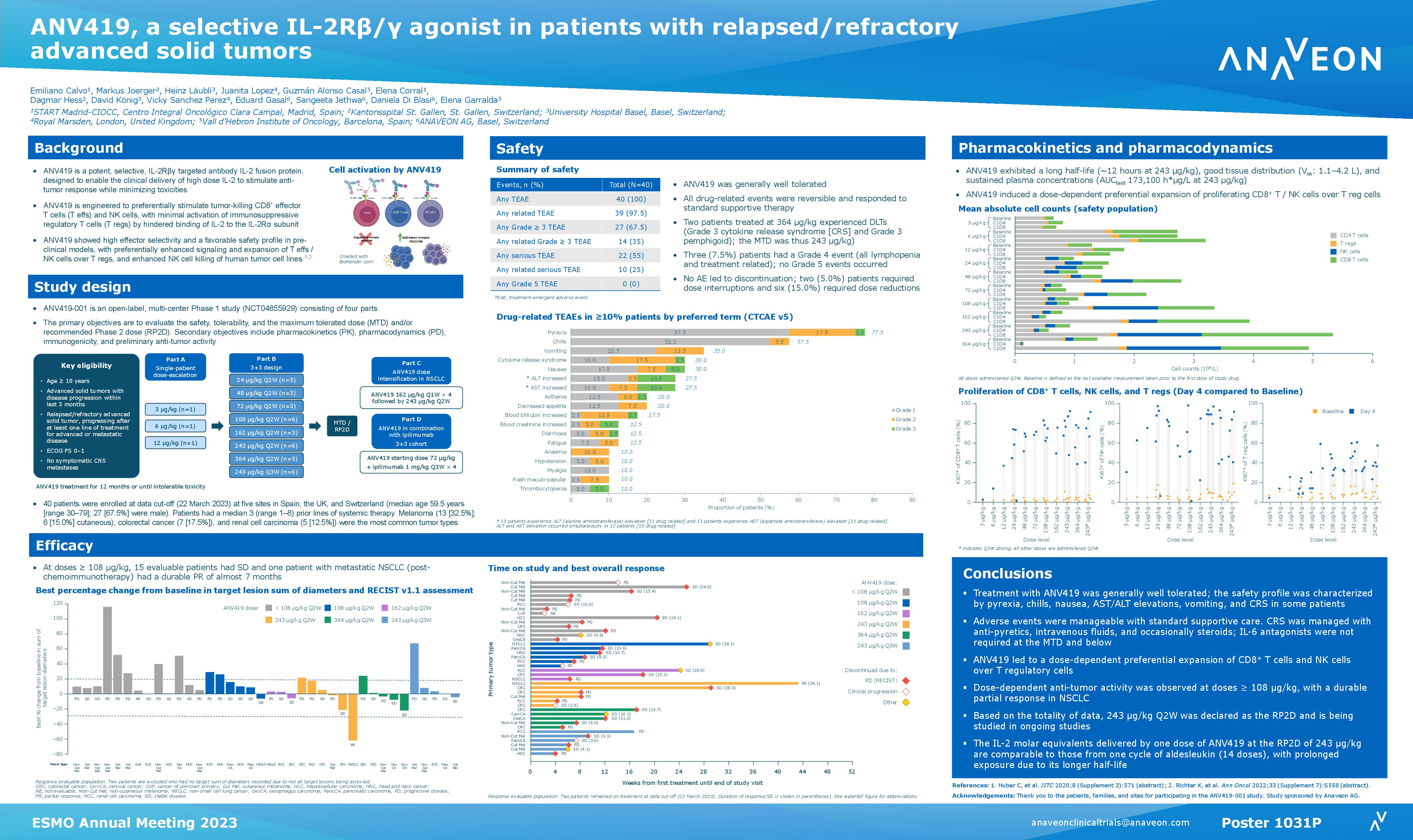
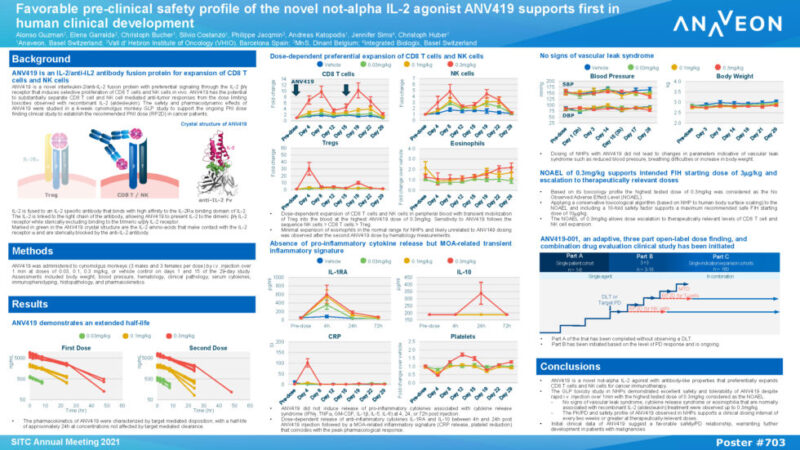
Non-Human Primate (NHP) data presented demonstrate that ANV419 has excellent safety and tolerability and selectively proliferates CD8 T cells and natural killer cells. Despite rapid intravenous injection over 1 minute, there were no signs of vascular leak syndrome, cytokine release syndrome or eosinophilia that are normally associated with recombinant IL-2 (aldesleukin) treatment up to the highest treated dose, with the highest dose of 0.3mg/kg considered the No Observed Adverse Effect Level (NOAEL). This favorable pharmacokinetic (PK)/pharmacodynamic (PD) and safety profile of ANV419 in NHPs supports a clinical dosing interval of every two weeks or greater at therapeutically relevant doses.
Based on the safety and the strong pharmacodynamic effects demonstrated in NHPs, Anaveon is conducting a Phase I ascending dose study of ANV419 monotherapy in patients with advanced solid tumors to determine safety and identify a Recommended Phase II Dose (RP2D) for ANV419 treatment. Early clinical data of ANV419 are consistent with the NHP results and suggest an attractive safety/PD relationship, warranting further development in patients with malignant tumors. ANV419, a powerful and selective interleukin-2 (IL-2) agonist, has been designed to overcome known challenges with tolerability and selectivity of recombinant human IL-2.
The abstract is available on the SITC website and the accompanying poster will be on display on the SITC 2021 virtual meeting platform and also available on Anaveon’s website.
“We are highly encouraged by the data presented at SITC,” said Christoph Huber, Chief Scientific Officer of Anaveon. “ANV149 has shown a class leading safety profile and we look forward to presenting the first clinical data at the American Association for Cancer Research (AACR) meeting in April 2022 and to exploring the drug’s efficacy in a range of cancer indications, as well as in combination with other therapeutics.”
Details of the poster presentation:
Title: Favorable pre-clinical safety profile of the novel not-alpha IL-2 agonist ANV419 supports first in human clinical development (download poster here)
Poster Presentation: Poster #703 – The ePoster will be on display on the SITC 2021 virtual meeting platform from 7:00 am ET on Friday, November 12, 2021 and will be presented on both Friday, November 12, 2021 and Saturday November 13, 2021 between 7.00 am and 8.30 am ET.
Anaveon was founded in December 2017. The Company is developing selective IL-2 Receptor Agonists, which have the potential to therapeutically enhance a patient’s immune system to respond to tumors. In the body, human IL-2 stimulates a type of immune cell, called a T-cell, to multiply and become activated. Activated T-cells are able to attack tumors and, consistent with this approach, human IL-2 is already approved as a therapeutic for the treatment of metastatic melanoma and renal cancer; however, due to lack of specificity, human IL-2 has severe, dose-limiting side effects and a short half-life that requires frequent infusions. The lead compound, ANV419, is designed to preferentially signal through the IL-2 beta/gamma receptor and therefore overcome known challenges of human IL-2. This novel type of therapeutic, if approved, could potentially have a wide utility in oncology, including in combination with cell therapies, vaccines, checkpoint inhibitors and radiotherapy.
ENDS
JW Communications
Julia Wilson
Tel: +44 (0)7818 430877
Email: julia.wilson@anaveon.com
About Anaveon:
Anaveon is a clinical stage, biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.