Anaveon doses first patient in a Phase I/II Study of ANV419 as monotherapy or in combination with check point inhibitors in patients with advanced melanoma
— OMNIA-1 is a Phase I/II study to determine the efficacy of ANV419 as monotherapy and in combination with anti-PD1 or anti-CTLA4 antibodies in advanced melanoma —
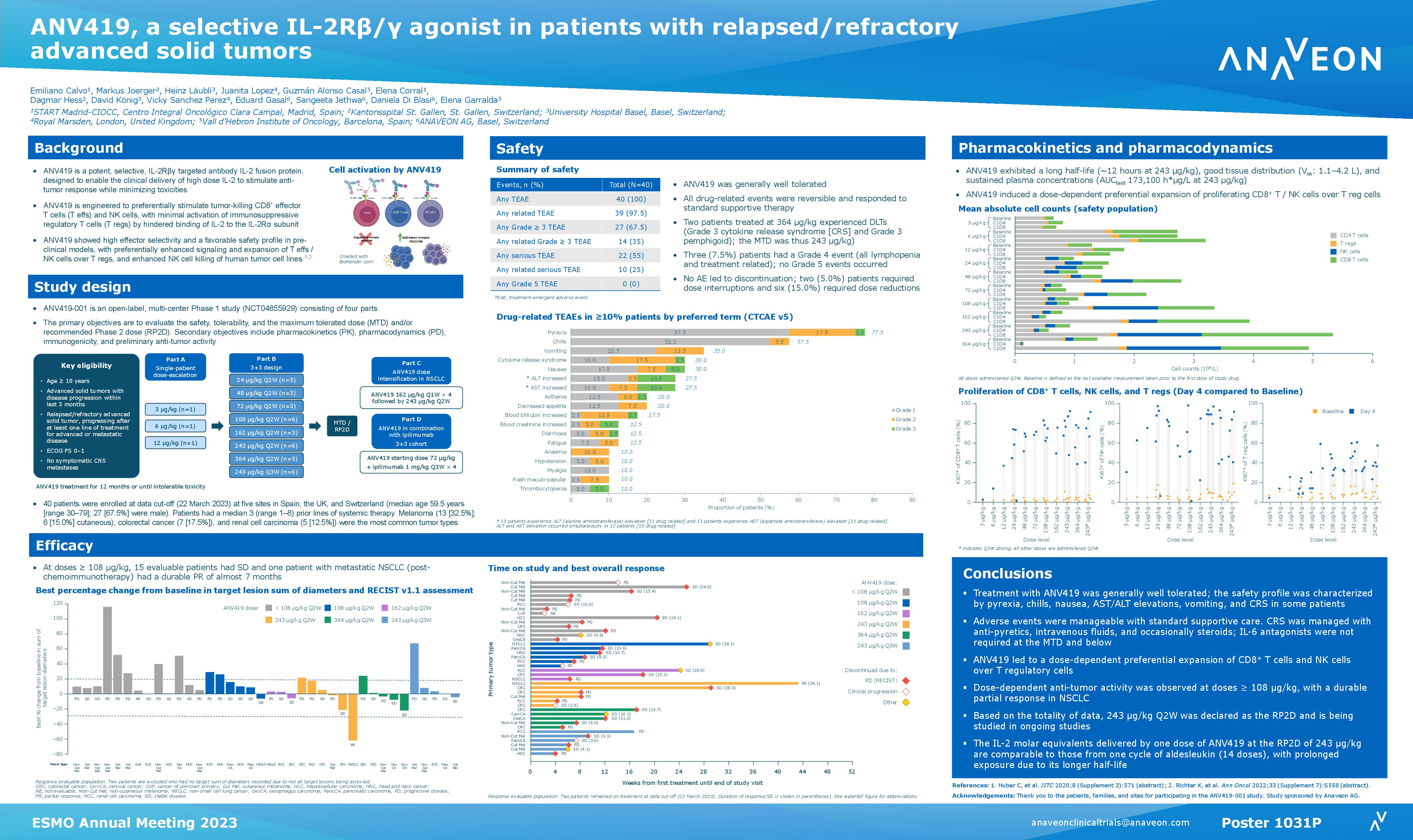
Basel, February 2, 2023 – Anaveon, a clinical stage, immuno-oncology company, today announced the first patient has been dosed in the OMNIA-1 study – a Phase I/II study assessing the efficacy and safety of ANV419 for the treatment of advanced melanoma. ANV419 is a powerful, IL-2Rbeta selective IL-2 agonist, which has been specifically designed to enable the delivery of high dose IL-2.
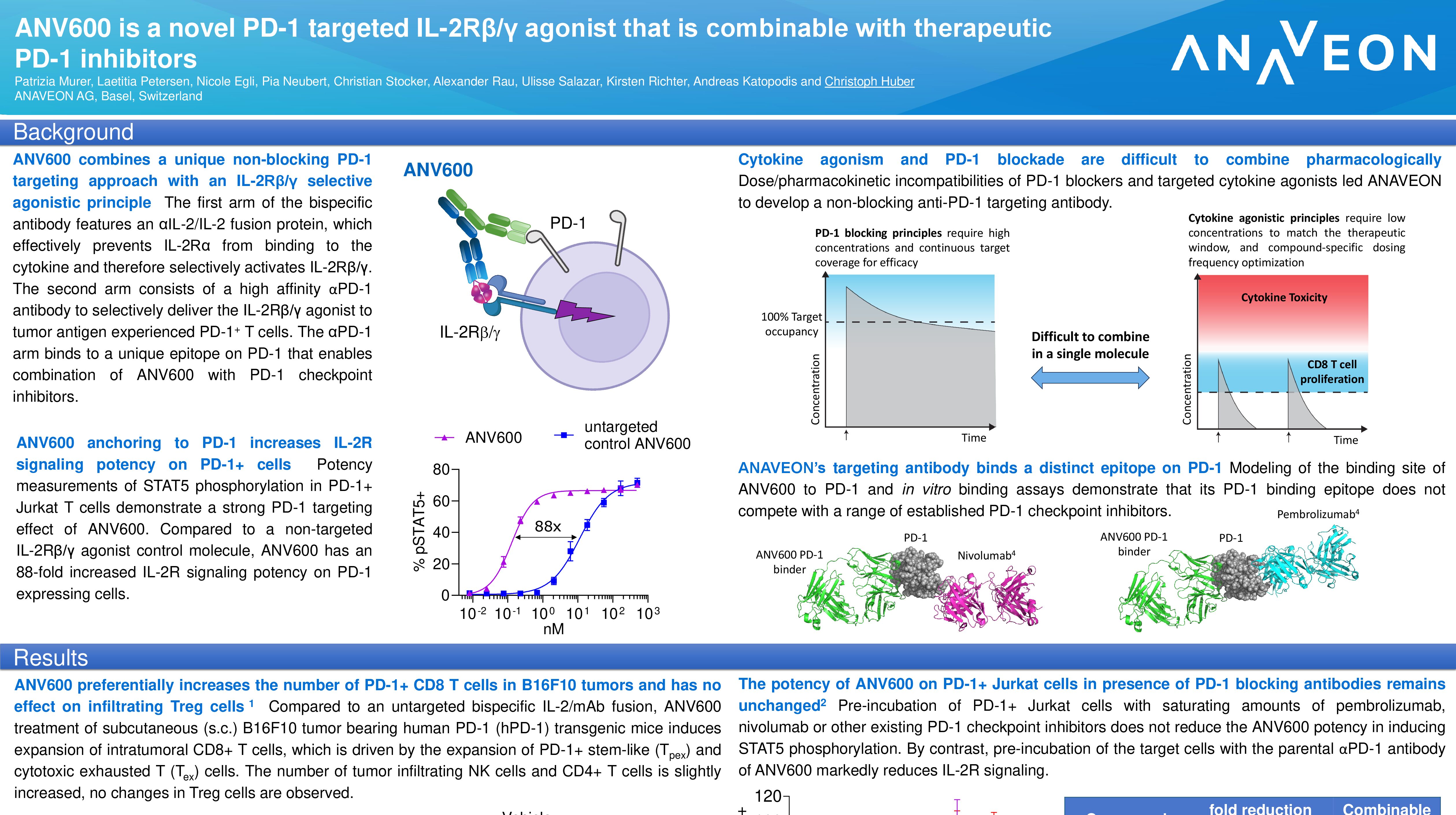
“In the first-in-human study, the data demonstrate the ability of ANV419 to be delivered at high molar equivalents of IL-2 in a tolerable and convenient way, with preferential stimulation and expansion of NK and CD8+ T cells,” said Eduard Gasal, MD, Chief Medical Officer at Anaveon. “We are excited to continue the development of our next-generation IL-2 in patients with advanced melanoma.”
In the past decade, there has been steady progress in the development of targeted therapy and immunotherapy for metastatic cutaneous melanoma with a substantial increase in the 5‑year overall survival (OS) rates from less than 10% to up to 50%. Despite substantial progress, 30% to 70% of patients do not respond to initial anti-programmed death 1/ligand 1 (PD-1/L1) therapy, and approximately 25% eventually progress. Thus, the overall outlook for patients with metastatic cutaneous melanoma remains challenging and the development of new effective and tolerable therapy is needed (1).
About the OMNIA-1 study
This global, open label, randomized, parallel arm, Phase I/II study (ANV419-101) will enrol up to 130 patients with advanced cutaneous melanoma. The study consists of a monotherapy dose expansion part, followed by a combination part of ANV419 with anti-PD1 or anti-CTLA-4 in patients who have progressed on or following standard of care immunotherapy (2).
Anaveon expects to report initial safety and efficacy data of the OMNIA-1 study by early 2024.
Anaveon is conducting several Phase I/II trials in parallel in solid tumors and hematological malignancies. In addition, Anaveon continues its work in developing follow-on compounds to expand on the success of ANV419 by delivering the IL-2 agonist to tumor fighting cells and expand into less immunogenic tumors. The Company is building on its cytokine engineering expertise with preclinical-stage programs harnessing the power of cytokines for therapeutic purposes.
(1) Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971-984.
(2) ClinicalTrials.gov, NCT05578872
ENDS
Enquiries
JW Communications
Julia Wilson
Email: julia.wilson@anaveon.com
Tel: +44 (0)7818 430877
About Anaveon
Anaveon is a clinical stage, biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.