Anaveon Presents Trial in Progress Data for OMNIA-1 at the American Society of Clinical Oncology 2023 Annual Meeting

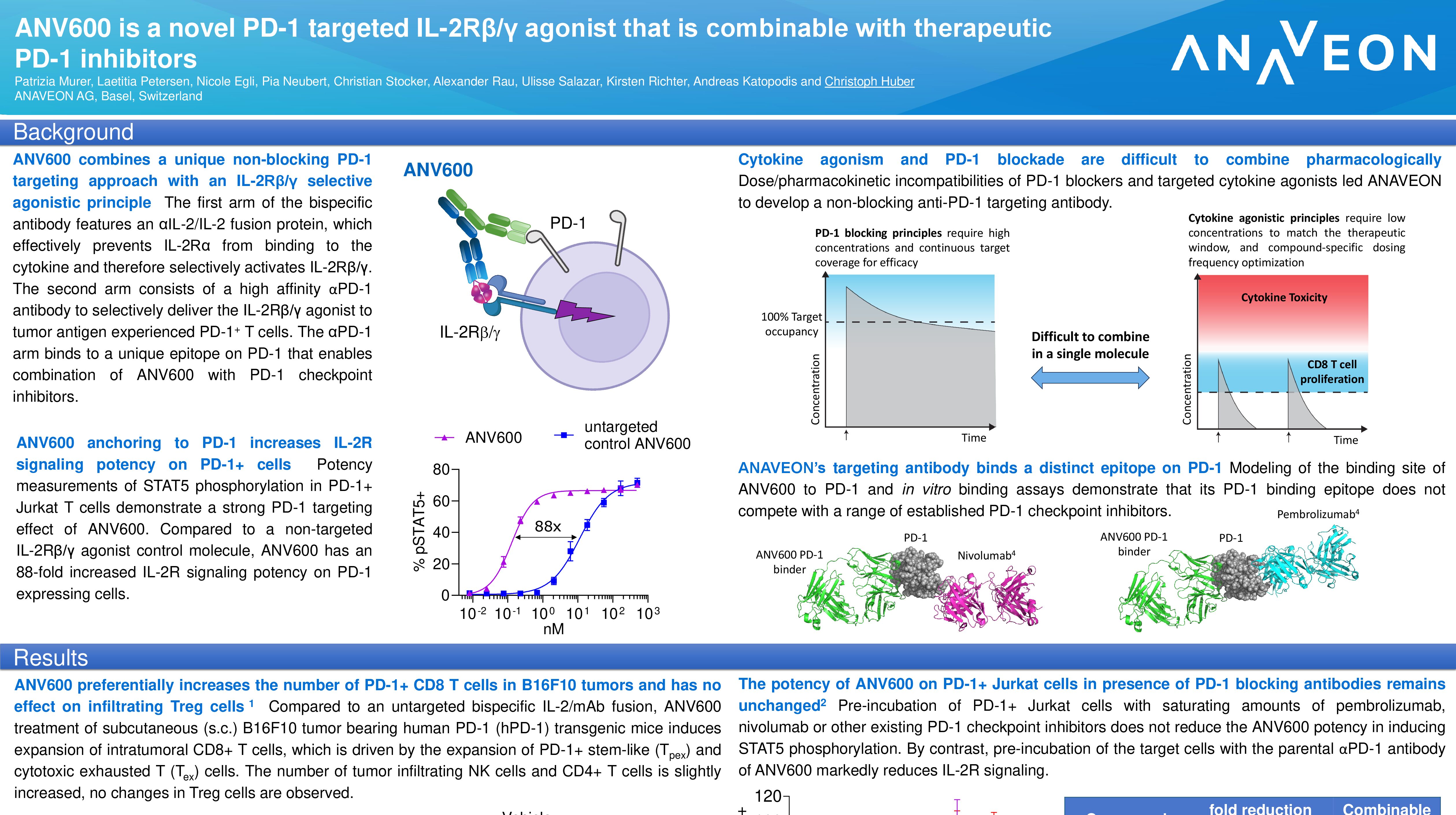
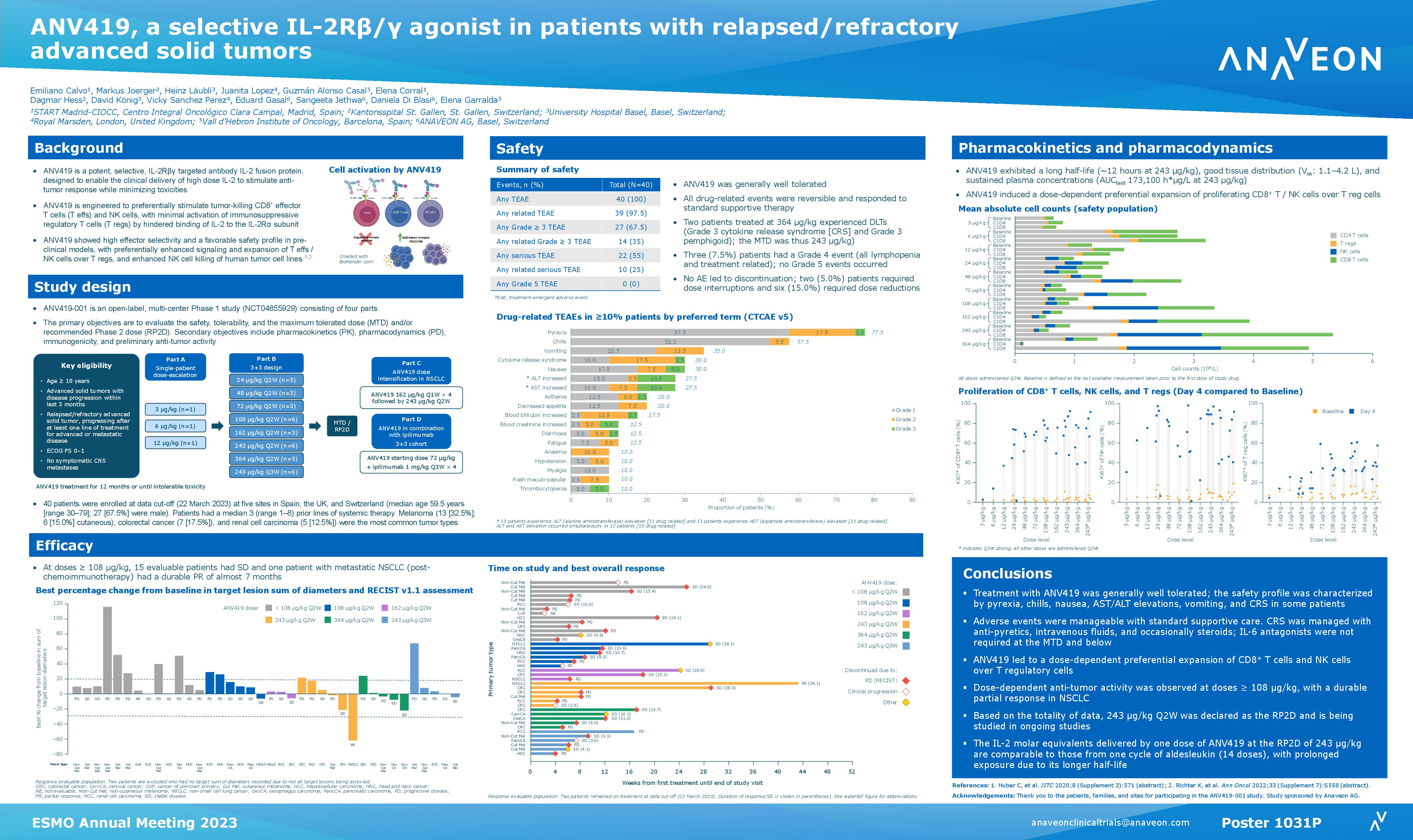
Basel, June 3, 2023 – OMNIA-1, a Phase I/II study of ANV419, an IL- 2R-βγ targeted antibody-IL-2 fusion protein, as monotherapy or in combination with anti-PD-1 or anti-CTLA-4 antibodies, in patients with advanced melanoma (download poster here).