Anaveon presents updated data from the Phase I/II study of ANV419 during the SITC Annual Meeting
— ANV419 delivers high dose IL-2 to patients with good safety and tolerability —
— Deepening of tumor response with continued ANV419 —
Basel, November 10, 2022 – Anaveon, a clinical stage, immuno-oncology company, today announced updated clinical data from the ongoing Phase I study of ANV419 in patients with solid tumors at the Society for Immunotherapy of Cancer (SITC) 37th Annual Meeting, being held from November 8–12, 2022, at the Boston Convention and Exhibition Center in Boston, MA.
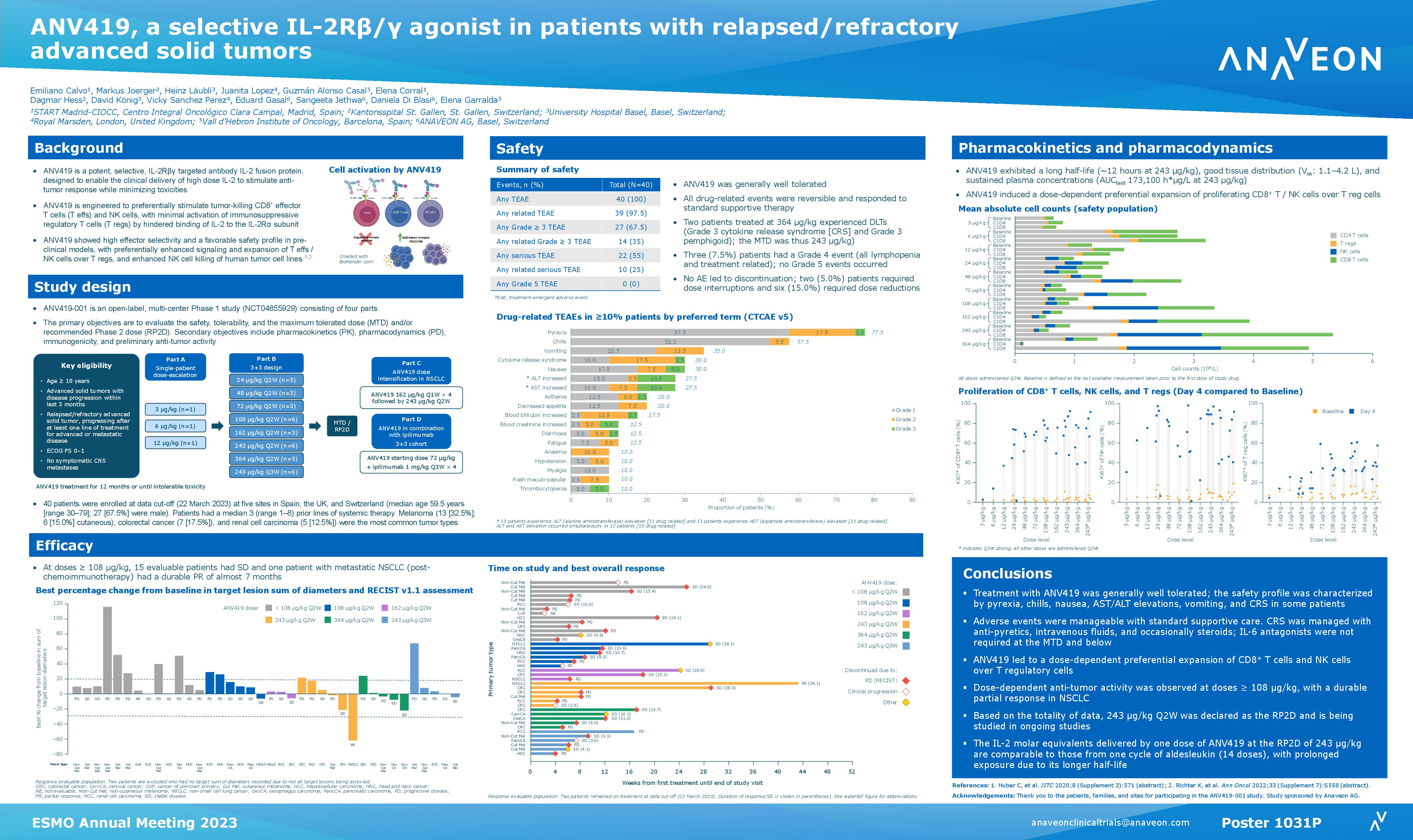
In the ongoing study, 29 patients in 10 dosing cohorts with different cancers progressing after standard therapy, received ANV419 monotherapy once every 14 days at doses up to 364 µg/kg in two-week cycles as an intravenous infusion over 15 minutes. ANV419 is a powerful and selective IL-2 agonist, which has been specifically designed to enable the delivery of high dose IL-2 to patients, with a favourable safety and tolerability profile.
In the study, ANV419 was generally well tolerated, and all drug related events were manageable, reversible, and responsive to supportive care therapy. The most common drug related AEs were low grade (G1 or G2) fever, chills, vomiting and fatigue. No patients have withdrawn from the study due to AEs and no dose limiting toxicities were observed up to and including 243 µg/kg.
In this heavily pre-treated population, 5 patients continue to receive treatment. At ANV419 doses ≥108 µg/kg, 66% of patients achieved at least disease stabilization (9 SD, 1 PR). One patient who continues ANV419 treatment, has a confirmed Partial Response (as per RECISTv1.1) with 31% tumor shrinkage after 2 weeks of ANV419 and a sustained and deepening response of 56% shrinkage at 6 cycles (12 weeks) of ANV419.
Pharmacodynamic evaluation of ANV419 on day 4 post-dosing (cycle 1 and 2) showed a selective and dose dependent proliferation of CD8+ T and NK cells, with a lower increase of proliferating Tregs. ANV419 half-life increases up to 28 hours with increasing doses and ANV419 concentration is overall stable between cycles.
Anaveon has initiated Ph I/II studies to demonstrate efficacy of ANV419 in metastatic melanoma and multiple myeloma.
Dr. Elena Garralda at the Hospital Universitari Vall d’Hebron in Barcelona, and lead investigator on the study said, “These early clinical data are encouraging and I believe ANV419 has the potential to become an important component of therapy for patients with cancer.”
Preclinical data for ANV419 in combination with checkpoint inhibitors that builds on the data presented at the European Society for Medical Oncology (ESMO) Congress 2022 in September were also presented at SITC. These continue to demonstrate broad activity of ANV419 on effector cells, supporting the initiation of Phase II studies assessing ANV419 treatment in indications in which CD8 T cells and NK cells are involved in tumor resolution as well as supporting combination studies with checkpoint inhibitors and treatments acting through antibody-dependent cellular cytotoxicity.
“It is very exciting to see the continued safety and tolerability of ANV419 at higher doses, demonstrating the ability of ANV419 to deliver high molar equivalents of IL-2 in a tolerable and convenient way. Importantly, tumor response continues to deepen,” added Christoph Bucher, MD, Chief Medical Officer of Anaveon. “We have initiated our first Phase II trial, investigating the efficacy of ANV419 in patients with cutaneous melanoma and we look forward to starting our Phase II study in Multiple Myeloma, whilst also broadening our pipeline with therapies that we expect will be effective as both standalone and combination therapies.”
Abstracts are available on the SITC website and the accompanying posters will be available in the publications section of Anaveon’s website.
Details of the poster presentations are:
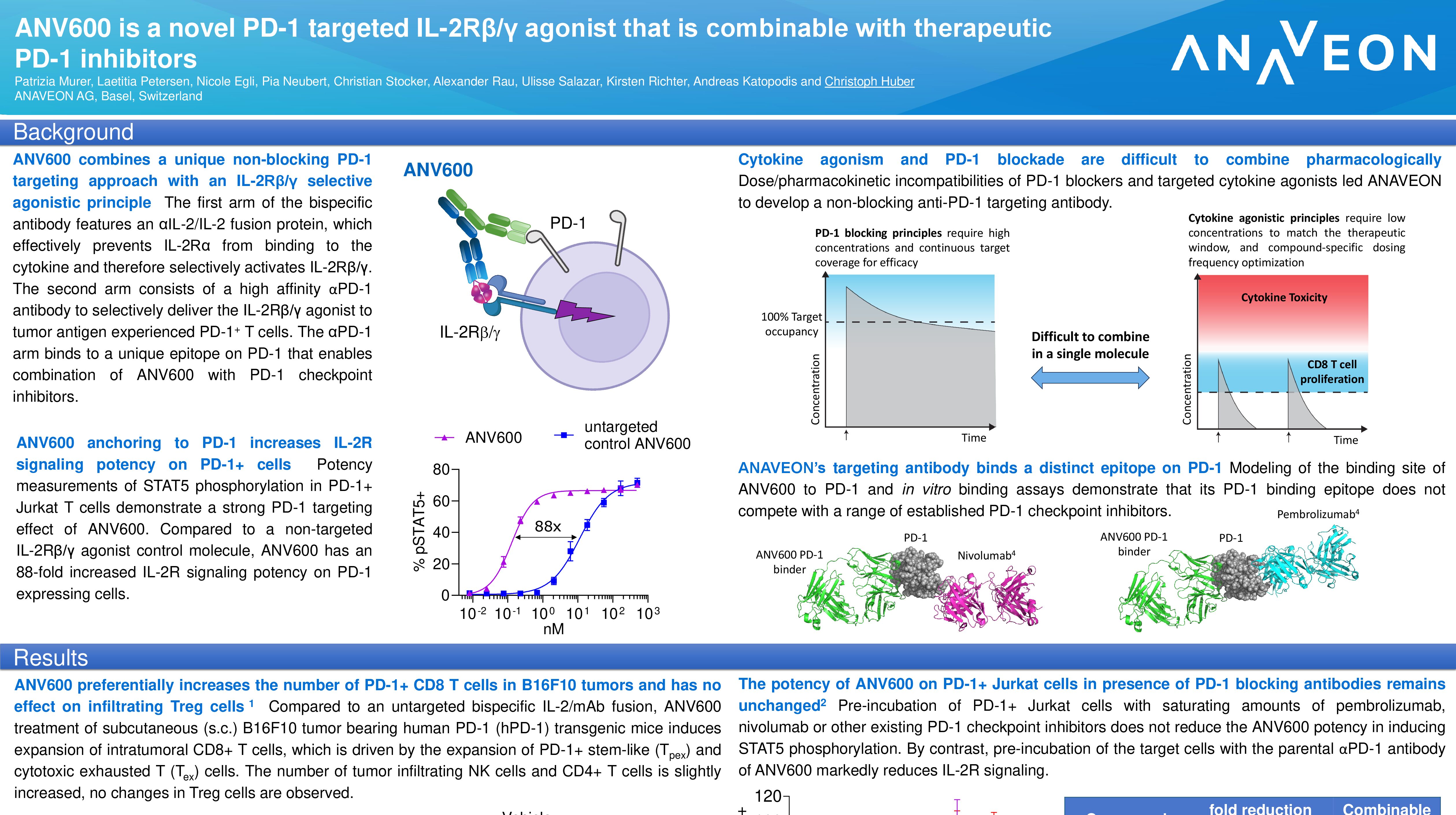
Title: Phase I dose escalation study in patients with advanced solid tumors with ANV419, a novel fusion protein selective for IL-2Rβ/γ (download poster 631 here)
Authors: Christoph Bucher, MD; Guzman Alonso, Dr; Juanita Lopez; Emiliano Calvo; Markus Joerger, MD; Vicky Sanchez Perez, MD; Elena Corral, MD; Daniela Di Blasi, PhD; Kirsten Richter, PhD; Christoph Huber, PhD; Julie Mouton; Silvio Costanzo; Sangeeta Jethwa, MD; Elena Garralda, MD; Heinz Läubli, MD PhD
Presenter: Daniela Di Blasi, PhD
Abstract Number: 631
Date & Time: Thursday, November 10, 2022 from 9 a.m. to 9 p.m. EST
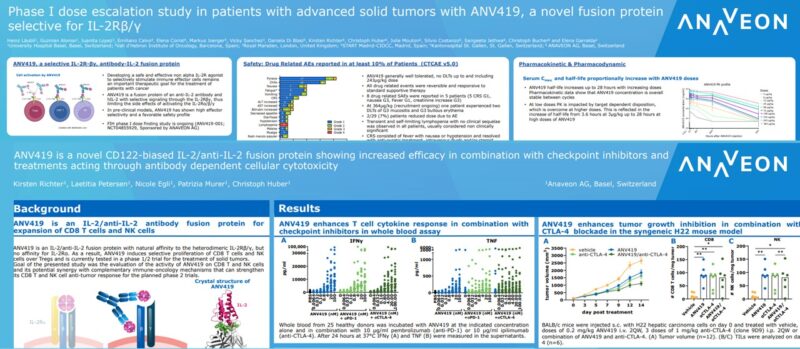
Title: ANV419 is a novel CD122-biased IL-2/anti-IL-2 fusion protein showing increased efficacy in combination with checkpoint inhibitors and treatments acting through antibody dependent cellular cytotoxicity (download poster 1099 here)
Authors: Christoph Huber, PhD; Kirsten Richter, PhD; Laetitia Petersen; Nicole Egli; Patrizia Murer, PhD
Presenter: Christoph Huber, PhD
Abstract Number: 1099
Date & Time: Thursday, November 10, 2022 from 9 a.m. to 9 p.m. EST
Anaveon is undertaking a Phase I/II study to evaluate the safety, dosing and clinical activity of its lead program, ANV419, a powerful and selective interleukin-2 (IL-2) agonist in patients with solid tumors. The Company is pursuing multiple parallel Phase II programs in order to explore the full therapeutic potential of ANV419. In addition, Anaveon continues its work in developing follow-on compounds to expand on the success of ANV419 by delivering the IL-2 agonist to tumor fighting cells and thus expand the therapeutic potential into less immunogenic tumors. Alongside this, the Company is building on its cytokine engineering expertise with preclinical-stage programs harnessing the power of cytokines for therapeutic purposes.
ENDS
Enquiries
JW Communications
Julia Wilson
Email: julia.wilson@anaveon.com
Tel: +44 (0)7818 430877
About Anaveon:
Anaveon is a clinical stage, biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.