Anaveon to present new data from the Phase I/II study of ANV419 at the ESMO Congress 2022
Basel, July 29, 2022 – Anaveon today announced that it will present new clinical data from the ongoing Phase I/II study of ANV419 in patients with solid tumors, as well as new preclinical data further elucidating the mode of action of this powerful and selective interleukin-2 (IL-2) agonist, in a poster presentation at the European Society for Medical Oncology (ESMO) Congress 2022, taking place in Paris, September 9 – 13, 2022. Abstracts will be available online starting 00.05 CEST on Monday, September 5, 2022.
Details of the poster presentations are:
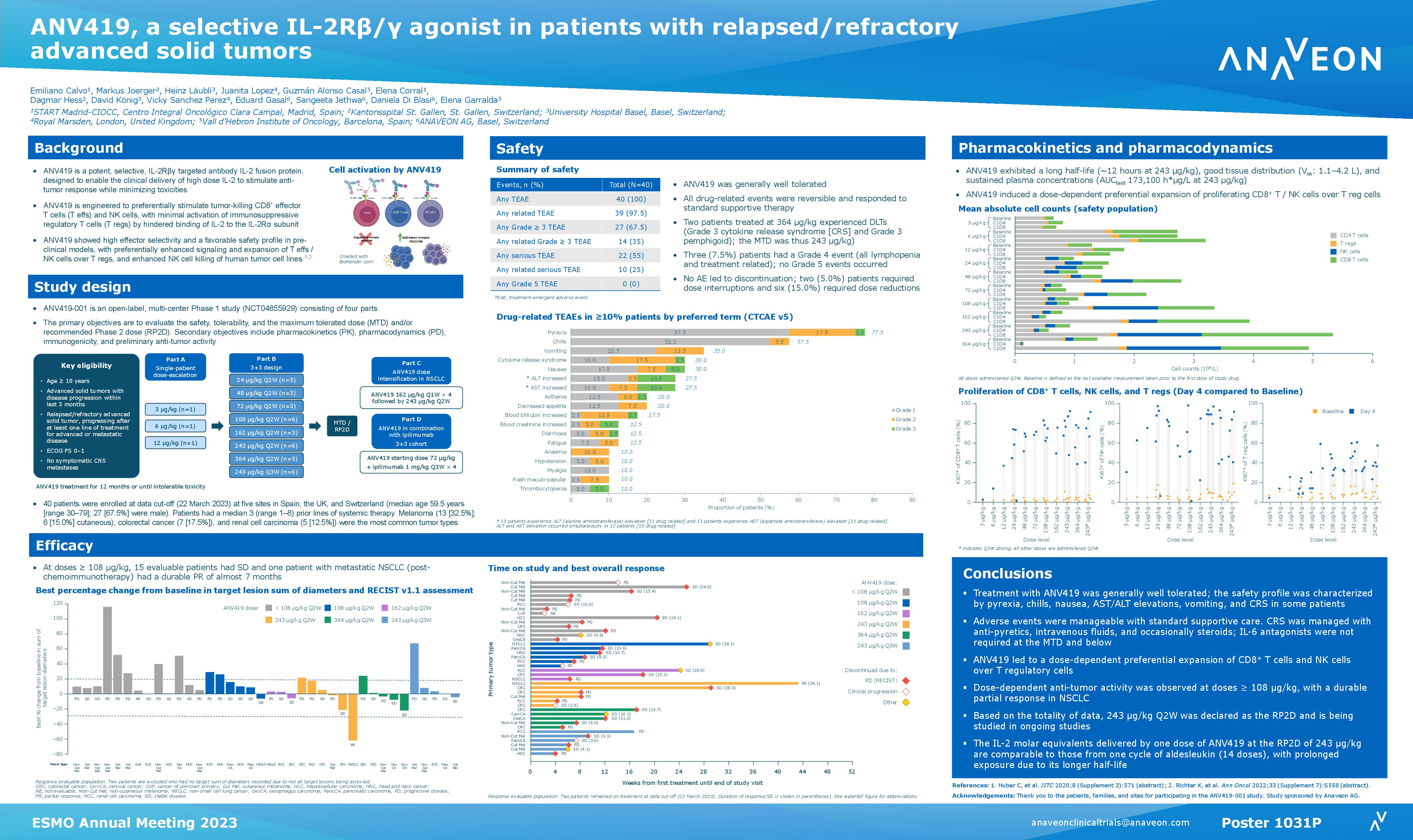
Abstract Title: “ANV419, a selective IL-2R-beta-gamma targeted antibody-IL-2 fusion protein, in patients with advanced solid tumors, a phase I/II study” (download poster 749P here)
Presentation Number: 479P Location: Poster Area Hall 4
Authors: H. Läubli, G. Alonso, J. Lopez, E. Calvo, M. Jörger, V. Sanchez, D. Di Blasi, A. Nair, K. Richter, Ch Huber, J Mouton, S. Costanzo, S. Jethwa, Ch Bucher and E. Garralda Date/Time: 12 September 2022 at 9:00 CEST – 18:30 CEST
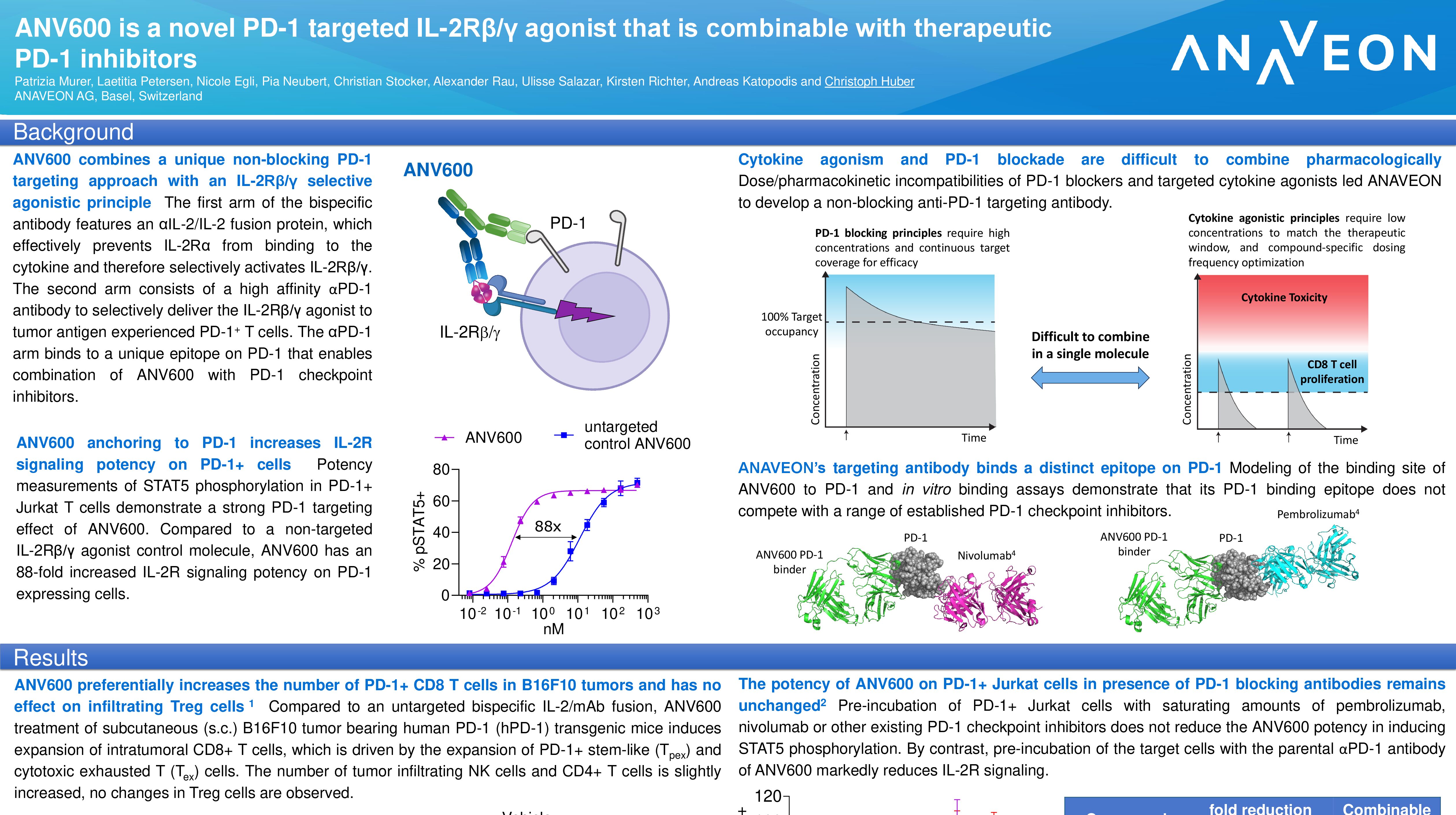
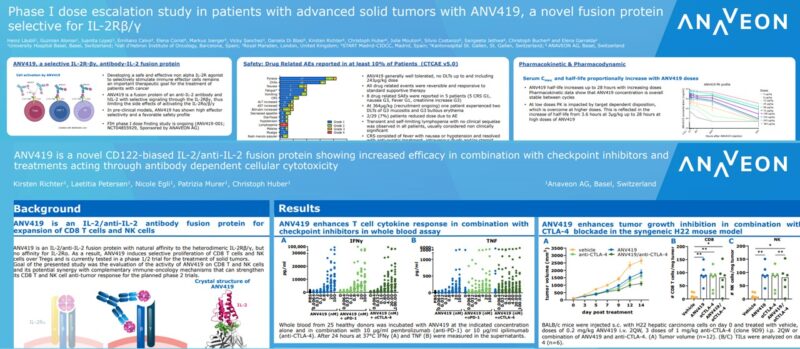
Abstract Title: “ANV419 is a novel CD122-biased IL-2/anti-IL-2 fusion protein with potent CD8 T cell and NK cell stimulating capacity that shows additive efficacy in combination with checkpoint inhibitors and treatments acting through antibody dependent cellular cytotoxicity” (download poster 39P here)
Presentation Number: 39P Location: Poster Area Hall 4
Authors: K. Richter, N. Egli, L. Petersen, P. Murer, A. Katopodis and Ch. Huber Date/Time: 11 September 2022 at 9:00 CEST – 18:30 CEST Please visit the ESMO website here for more information. Anaveon is undertaking a Phase I/II study to evaluate the safety, dosing and clinical activity of its lead program, ANV419, a powerful and selective interleukin-2 (IL-2) agonist in patients with solid tumors. The Company is pursuing multiple parallel Phase II programs in order to explore the full therapeutic potential of ANV419. In addition, Anaveon continues its work in developing follow-on compounds to expand on the success of ANV419 by delivering the IL-2 agonist to tumor fighting cells and thus expand the therapeutic potential into less immunogenic tumors. Alongside this, the Company is building on its cytokine engineering expertise with preclinical-stage programs harnessing the power of cytokines for therapeutic purposes.
ENDS
Enquiries
JW Communications
Julia Wilson
Tel: +44 (0)7818 430877
Email: julia.wilson@anaveon.com
About Anaveon:
Anaveon is a clinical-stage biopharmaceutical company, based in Switzerland, that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology. For further information please visit the Company’s website at: www.anaveon.com.