Anaveon announces presentation at the 2020 Society for Immunotherapy of Cancer (SITC) Annual Meeting

— ANV419 is a unique approach to no-alpha IL-2 agonists by taking advantage of a highly selective antibody which binds the same site as the alpha receptor and is able to completely abolish its binding —
Basel, November 10, 2020 – Anaveon, an immuno-oncology company, today announced that it will present a poster at the Society for Immunotherapy of Cancer’s (SITC) 35th Virtual Annual Meeting being held from Monday, November 9, 2020 to Saturday, November 14, 2020.
The abstract is available on the SITC website and the accompanying poster will be available in the Virtual Poster Hall open from 8.00 a.m. EST on Monday, November 9, until the Virtual Poster Hall closes on December 31, 2020 and is also available on Anaveon’s website.
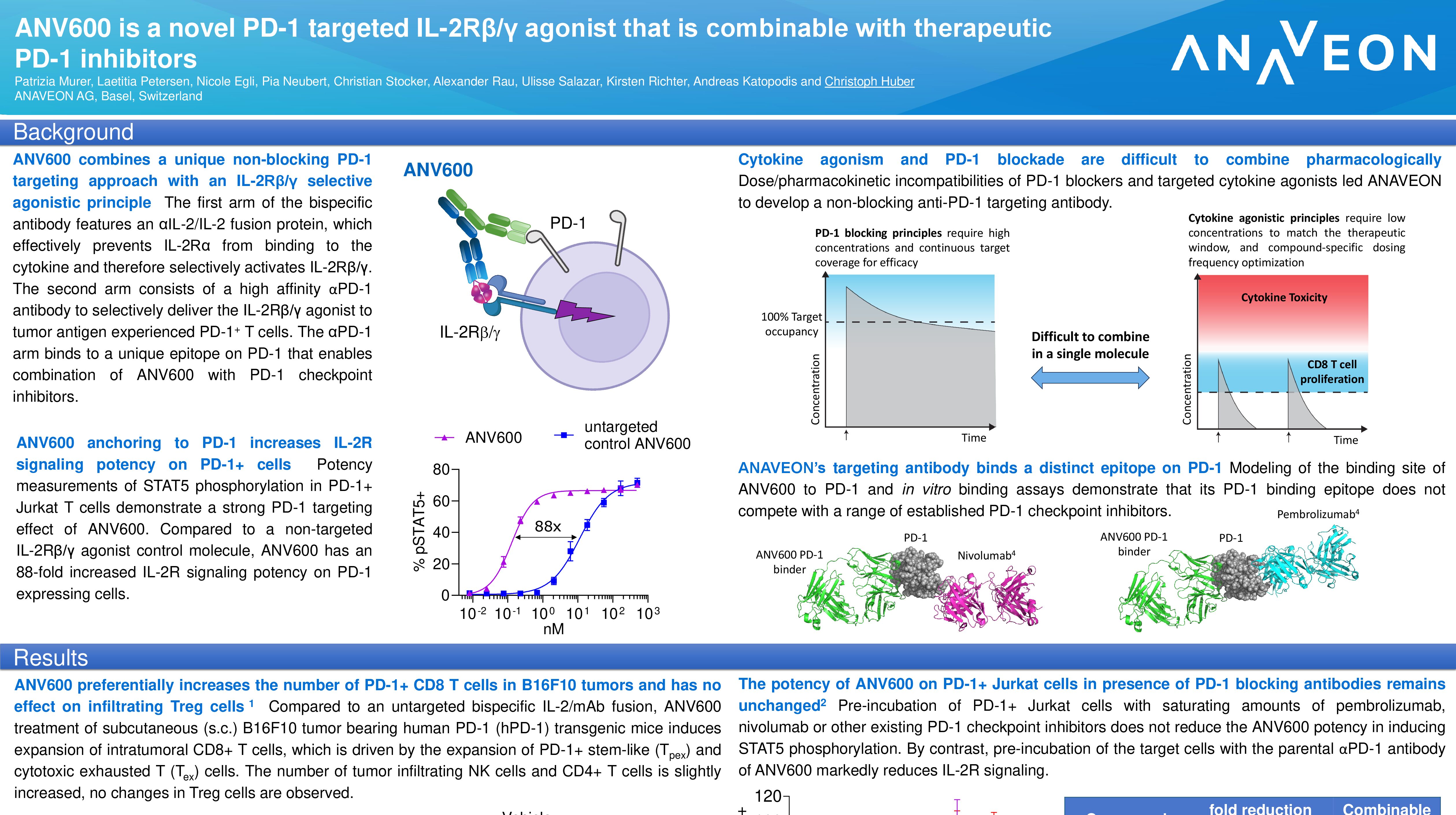
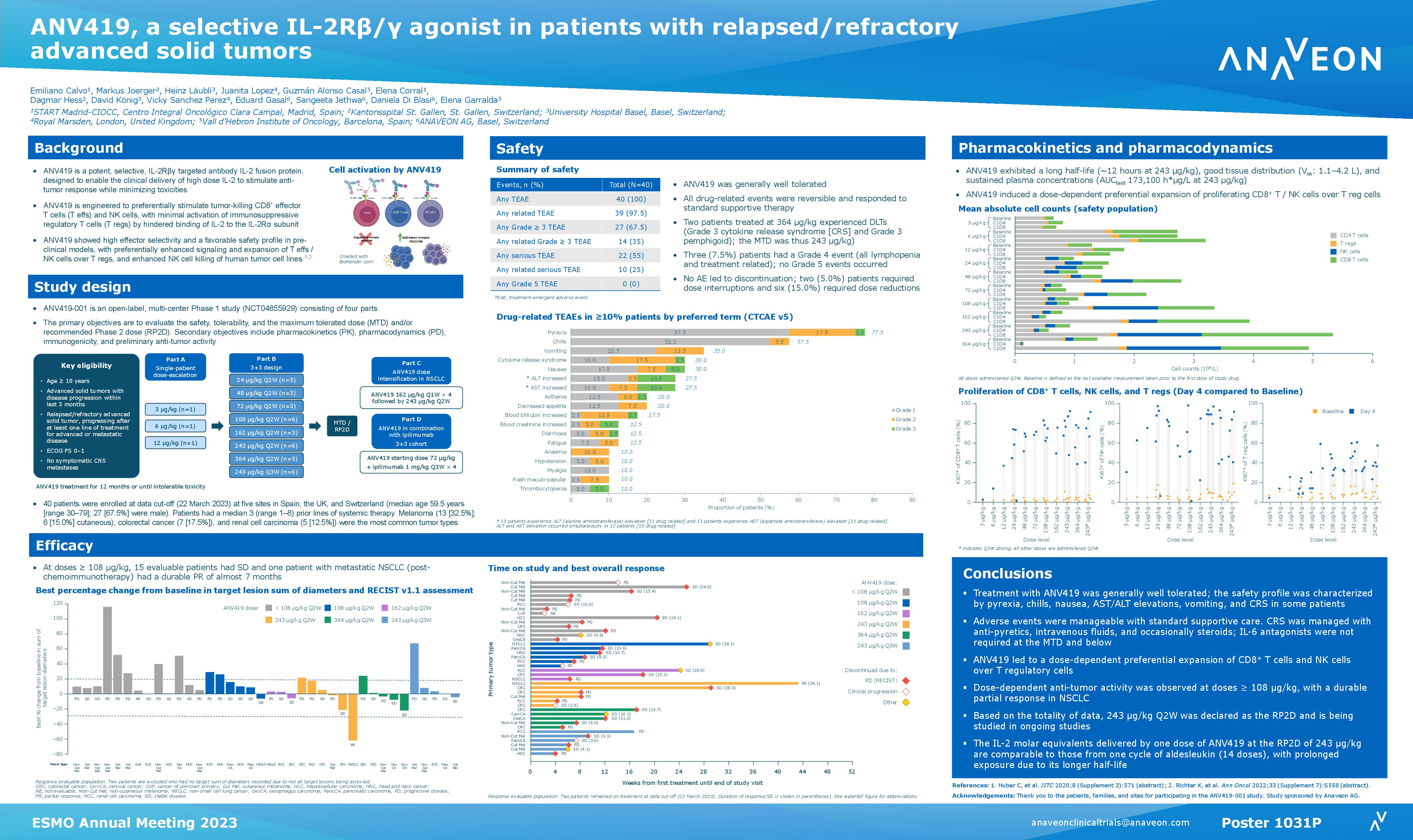
ANV419, Anaveon’s lead programme, is a powerful and selective interleukin-2 (IL-2) agonist that is in late stage preclinical development for the treatment of cancer. It represents a unique approach to no-alpha IL-2 agonists by taking advantage of a highly selective antibody which binds to the same site as the IL-2 alpha receptor and is able to completely abolish its binding.
The data demonstrates that ANV419 presents IL-2 to the beta/gamma IL-2 receptor with similar potency as native IL-2 and thus provides a natural growth signal to anti-tumor CD8+ T cells and natural killer (NK) cells while it avoids promoting pro-tumor regulatory T cells. The compound is a high molecular weight biologic that behaves like an antibody and has an outstanding safety profile in non-human primates.
“We are excited by the data demonstrating that ANV419 has a unique approach to no-alpha IL-2 agonists by taking advantage of a highly selective antibody which binds the same site as the alpha receptor and is able to completely abolish its binding,” said Christoph Huber, Chief Scientific Officer of Anaveon. “With its high level of activity on anti-tumor immune cells and excellent safety profile we look forward to starting our first clinical study in Q1 2021 and to exploring ANV419’s applicability in a range of cancer indications, as well as in combination with other therapeutics.”
Details of the poster presentation:
Title: ANV419 is a novel CD122-selective IL-2/anti-IL-2 antibody fusion protein with potent CD8 T cell and NK cell stimulatory function in vitro and in vivo (download poster here)
Live Q&A: Poster #571 – Wednesday, Nov. 11 from 5:15-5:45 p.m. EST and Friday, Nov. 13 from 4:40-5:10 p.m. EST
Anaveon was founded in December 2017 by Andreas Katopodis, previously Director at the Autoimmunity, Transplantation & Inflammation Group at the Novartis Institutes for BioMedical Research and Onur Boyman, Professor and Chair in the Department of Immunology at the University of Zurich. The Company is developing selective IL-2 Receptor Agonists, a type of protein that could therapeutically enhance a patient’s immune system to respond to tumors. In the body, human IL-2 stimulates a type of immune cell, called a T-cell, to multiply and become activated. Under certain situations, T-cells are able to attack tumors and, consistent with this, human IL-2 is already approved as a therapeutic for the treatment of metastatic melanoma and renal cancer. The lead compound, ANV419, is designed to overcome known challenges with human IL-2. These include severe, dose-limiting side effects and a short half-life that requires frequent infusions. This type of drug, if approved, could potentially have a wide utility in oncology, including in combination with cell therapies, vaccines, checkpoint inhibitors and radiotherapy.
ENDS
Enquiries:
Anaveon AG
Andreas Katopodis, CEO
Email: contact@anaveon.com
JW Communications
Julia Wilson
Email: julia.wilson@anaveon.com
About Anaveon:
Anaveon is a pre-clinical phase start-up based in Switzerland that develops biologics to modulate the function of cytokines and provide substantial therapeutic benefit to cancer patients. Our vision is to develop novel immune therapies benefiting patients suffering from a wide variety of diseases with immune pathology.